User:Sandbh/Alternate periodic tables redraft: Difference between revisions
The collocation of manganese with iron, nickel and cobalt is later seen in the modernised version of von Bichowsky’s table of 1918, in the unclassified section of this article.
The collocation of manganese with iron, nickel and cobalt is later seen in the modernised version of von Bichowsky’s table of 1918, in the unclassified section of this article.
The advantage of this form is that it emphasizes, to a create or lesser degree, that the elements form a continuous sequence; that said, continuous tables are harder to read, draw and memorize than the traditional rectangular form of periodic table
The advantage of this form is that it emphasizes, to a create or lesser degree, that the elements form a continuous sequence; that said, continuous tables are harder to , and memorize than the traditional rectangular form of periodic table
{{clear}}
{{clear}}
List of periodic table shapes

Since Dimitri Mendeleev formulated the periodic law in 1871, and published an associated periodic table of chemical elements, authors have experimented with varying shapes of periodic tables including for teaching, aesthetic or philosophical purposes. Some forms of periodic tables depict some relationships between chemical elements better than an in other tables.
Typology[edit]
Periodic table shapes may, for convenience, be classed as 1. short; 2. triangular; 3. medium; 4. long; 5. continuous (circular, spiral, lemniscate, or helical); 6. folding; or 7. spatial. Those that defy easy classification are counted as type 8. unclassified.
Short[edit]
Short tables have around eight columns. This form became popular following the publication of Mendeleev’s eight-column periodic table in 1871.
Also shown in this section is a modernized version of the same table.
Mendeleev and others who discovered chemical periodicity in the 1860s had noticed that when the elements were arranged in order of their atomic weights there was as an approximate repetition of physiochemical properties after every eight elements. Consequently, Mendeleev organized the elements known at that time into a table with eight columns. He used the table to predict the properties of then unknown elements. While his hit rate was less than 50% it was his successes that propelled the widespread acceptance of the idea of a periodic table of the chemical elements.[2] The eight-column style remains popular to this day, most notably in Russia, Mendeleev’s country of birth.
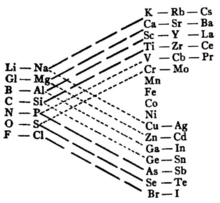
An earlier attempt by Newlands, an English chemist, to present the nub of the same idea to the London Chemical Society, in 1866, was unsuccessful;[3] members were less than receptive to theoretical ideas, as was the British tendency at the time.[4] He referred to his idea as the Law of Octaves, at one point drawing an analogy with an eight-key musical scale.
John Gladstone, a fellow chemist, objected on the basis that Newland’s table presumed no elements remained to be discovered. “The last few years had brought forth thallium, indium, caesium, and rubidium, and now the finding of one more would throw out the whole system.”[3] He believed there was as close an analogy between the metals named in the last vertical column as in any of the elements standing on the same horizontal line.
Fellow English chemist Carey Foster humorously inquired of Newlands whether he had ever examined the elements according to the order of their initial letters. Foster believed that any arrangement would present occasional coincidences, but he condemned one which placed so far apart manganese and chromium, or iron from nickel and cobalt.
The advantages of the short form of periodic table are its compact size and that it shows the relationships between main group elements and transition metal groups; its disadvantages are that it fails to accomodate the electron configuration arrangements of the elements and that it appears to group dissimilar elements such as chorine and manganese together.
Triangular[edit]
Triangular tables have column widths of 2-8-18-32 or thereabouts. An early example, appearing in 1882, was provided by Bayley.[5]
Through the use of connecting…
Read More: User:Sandbh/Alternate periodic tables redraft: Difference between revisions





